Review Paper on Azide-alkyne Click Chemistry of Polymers Chemical Reviews
In chemical synthesis, "click" chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is non a single specific reaction, but describes a style of generating products that follow examples in nature, which also generates substances by joining modest modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological weather condition: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, and various biomimetic applications. Still, they have been made notably useful in the detection, localization and qualification of biomolecules.
Click reactions occur in i pot, are not disturbed by water, generate minimal and inoffensive byproducts, and are "spring-loaded"—characterized by a high thermodynamic driving force that drives it quickly and irreversibly to high yield of a unmarried reaction product, with high reaction specificity (in some cases, with both regio- and stereo-specificity). These qualities make click reactions particularly suitable to the problem of isolating and targeting molecules in circuitous biological environments. In such environments, products appropriately need to be physiologically stable and any byproducts need to exist non-toxic (for in vivo systems).
Past developing specific and controllable bioorthogonal reactions, scientists take opened upwards the possibility of hitting particular targets in complex jail cell lysates. Recently, scientists take adjusted click chemistry for use in alive cells, for example using pocket-sized molecule probes that observe and attach to their targets past click reactions. Despite challenges of cell permeability, bioorthogonality, background labeling, and reaction efficiency, click reactions have already proven useful in a new generation of pulldown experiments (in which detail targets can be isolated using, for example, reporter molecules which bind to a certain column), and fluorescence spectrometry (in which the fluorophore is attached to a target of interest and the target quantified or located). More than recently, novel methods have been used to incorporate click reaction partners onto and into biomolecules, including the incorporation of unnatural amino acids containing reactive groups into proteins and the modification of nucleotides. These techniques correspond a office of the field of chemical biology, in which click chemistry plays a fundamental role by intentionally and specifically coupling modular units to various ends.
The term "click chemical science" was coined past K. Barry Sharpless in 1998, and was first fully described past Sharpless, Hartmuth Kolb, and M.G. Finn of The Scripps Research Institute in 2001.[ane] [2]
Background [edit]
Click chemistry is a method for attaching a probe or substrate of interest to a specific biomolecule, a procedure called bioconjugation. The possibility of attaching fluorophores and other reporter molecules has made click chemistry a very powerful tool for identifying, locating, and characterizing both quondam and new biomolecules.
One of the primeval and most of import methods in bioconjugation was to express a reporter on the same open up reading frame as a biomolecule of involvement. Notably, GFP was beginning (and still is) expressed in this way at the Northward- or C- terminus of many proteins. Still, this arroyo comes with several difficulties. For example, GFP is a very large unit of measurement and can often touch on the folding of the protein of interest. Moreover, by existence expressed at either terminus, the GFP adduct can also affect the targeting and expression of the desired poly peptide. Finally, using this method, GFP tin simply exist fastened to proteins, and not post-translationally, leaving other important biomolecular classes (nucleic acids, lipids, carbohydrates, etc.) out of reach.
To overcome these challenges, chemists take opted to proceed by identifying pairs of bioorthogonal reaction partners, thus assuasive the use of small exogenous molecules as biomolecular probes. A fluorophore can be attached to one of these probes to give a fluorescence signal upon binding of the reporter molecule to the target—just as GFP fluoresces when it is expressed with the target.
Now limitations emerge from the chemical science of the probe to its target. In order for this technique to be useful in biological systems, click chemistry must run at or near biological conditions, produce little and (ideally) non-toxic byproducts, accept (preferably) single and stable products at the same weather condition, and proceed quickly to high yield in i pot. Existing reactions, such every bit Staudinger ligation and the Huisgen ane,three-dipolar cycloaddition, take been modified and optimized for such reaction atmospheric condition. Today, research in the field concerns not only understanding and developing new reactions and repurposing and re-understanding known reactions, simply likewise expanding methods used to incorporate reaction partners into living systems, engineering novel reaction partners, and developing applications for bioconjugation.
Reactions [edit]
For a reaction to be considered a click reaction, information technology must satisfy sure characteristics:[3]
- modularity
- insensitivity to solvent parameters
- high chemical yields
- insensitivity towards oxygen and water
- regiospecificity and stereospecificity
- a big thermodynamic driving strength (>xx kcal/mol) to favor a reaction with a single reaction product. A distinct exothermic reaction makes a reactant "spring-loaded."
The process would preferably:
- have unproblematic reaction weather condition
- utilize readily bachelor starting materials and reagents
- utilize no solvent or use a solvent that is benign or hands removed (preferably water)
- provide uncomplicated production isolation by non-chromatographic methods (crystallisation or distillation)
- have high atom economy.
Many of the click chemical science criteria are subjective, and even if measurable and objective criteria could be agreed upon, information technology is unlikely that any reaction will be perfect for every state of affairs and application. However, several reactions have been identified that fit the concept better than others:[ clarification needed ]
- [3+two] cycloadditions, such as the Huisgen 1,3-dipolar cycloaddition, in particular the Cu(I)-catalyzed stepwise variant,[four] are oftentimes referred to simply as Click reactions
- Thiol-ene reaction[v] [half-dozen]
- Diels-Alder reaction and inverse electron demand Diels-Alder reaction[7] [viii]
- [4+i] cycloadditions between isonitriles (isocyanides) and tetrazines[9]
- nucleophilic substitution especially to small strained rings like epoxy[10] and aziridines
- carbonyl-chemical science-like formation of ureas but not reactions of the aldol type due to low thermodynamic driving force.
- improver reactions to carbon-carbon double bonds like dihydroxylation or the alkynes in the thiol-yne reaction.[3]
Copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) [edit]
The archetype[eleven] [12] click reaction is the copper-catalyzed reaction of an azide with an alkyne to class a 5-membered heteroatom band: a Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). The showtime triazole synthesis, from diethyl acetylenedicarboxylate and phenyl azide, was reported past Arthur Michael in 1893.[thirteen] After, in the heart of the 20th century, this family of 1,3-dipolar cycloadditions took on Huisgen'southward name subsequently his studies of their reaction kinetics and weather condition.
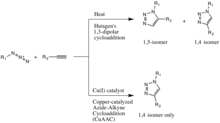
A comparing of the Huisgen and the copper-catalyzed Azide-Alkyne cycloadditions
The copper(I)-catalysis of the Huisgen 1,3-dipolar cycloaddition was discovered concurrently and independently by the groups of Valery Five. Fokin and K. Barry Sharpless at the Scripps Research Institute in California[14] and Morten Meldal in the Carlsberg Laboratory, Denmark.[xv] The copper-catalyzed version of this reaction gives only the 1,4-isomer, whereas Huisgen's non-catalyzed ane,three-dipolar cycloaddition gives both the 1,4- and 1,5-isomers, is slow, and requires a temperature of 100 degrees Celsius.[13]

The two-copper mechanism of the CuAAC catalytic cycle
Moreover, this copper-catalyzed "click" does not require ligands on the metal, although accelerating ligands such as tris(triazolyl)methyl amine ligands with diverse substituents have been reported and used with success in aqueous solution.[thirteen] Other ligands such as PPh3 and TBIA tin can besides exist used, fifty-fifty though PPh3 is liable to Staudinger ligation with the azide substituent. Cu2O in water at room temperature was constitute besides to catalyze the same reaction in 15 minutes with 91% yield.[sixteen]
The first reaction mechanism proposed included one catalytic copper atom; simply isotope, kinetic, and other studies have suggested a dicopper mechanism may exist more relevant.[17] [eighteen] [xix] [20] [21] Fifty-fifty though this reaction proceeds effectively at biological weather, copper in this range of dosage is cytotoxic. Solutions to this problem have been presented, such as using water-soluble ligands on the copper to enhance cell penetration of the catalyst and thereby reduce the dosage needed,[22] [23] [24] or to apply chelating ligands to further increase the effective concentration of Cu(I) and thereby decreasing the actual dosage.[25] [26] [27]
Although the Cu(I)-catalyzed variant was starting time reported past Meldal and co-workers for the synthesis of peptidotriazoles on solid back up, they needed more fourth dimension to find the full telescopic of the reaction and were overtaken by the publicly more than recognized Sharpless. Meldal and co-workers also chose not to characterization this reaction type "click chemical science" which allegedly caused their discovery to exist largely overlooked by the mainstream chemical society. Sharpless and Fokin independently described it as a reliable catalytic process offering "an unprecedented level of selectivity, reliability, and scope for those organic synthesis endeavors which depend on the cosmos of covalent links between diverse building blocks."
An analogous RuAAC reaction catalyzed by ruthenium, instead of copper, was reported by the Jia and Fokin groups in 2005, and allows for the selective production of 1,5-isomers.[28]
Strain-promoted azide-alkyne cycloaddition (SPAAC) [edit]
The Bertozzi grouping further developed 1 of Huisgen's copper-gratuitous click reactions to overcome the cytotoxicity of the CuAAC reaction.[29] Instead of using Cu(I) to activate the alkyne, the alkyne is instead introduced in a strained difluorooctyne (DIFO), in which the electron-withdrawing, propargylic, gem-fluorines act together with the band strain to greatly destabilize the alkyne.[30] This destabilization increasing the reaction driving force, and the desire of the cycloalkyne to relieve its band strain.

Scheme of the Strain-promoted Azide-Alkyne Cycloaddition
This reaction proceeds as a concerted [3+ii] cycloaddition in the same machinery as the Huisgen 1,3-dipolar cycloaddition. Substituents other than fluorines, such as benzene rings, are also allowed on the cyclooctyne.
This reaction has been used successfully to probe for azides in living systems, even though the reaction charge per unit is somewhat slower than that of the CuAAC. Moreover, because the synthesis of cyclooctynes often gives low yield, probe evolution for this reaction has not been equally rapid as for other reactions. But cyclooctyne derivatives such as DIFO, dibenzylcyclooctyne (DIBO) and biarylazacyclooctynone (BARAC) have all been used successfully in the SPAAC reaction to probe for azides in living systems.[31] [32] [33]
Strain-promoted alkyne-nitrone cycloaddition (SPANC) [edit]
Diaryl-strained-cyclooctynes including dibenzylcyclooctyne (DIBO) have also been used to react with 1,3-nitrones in strain-promoted alkyne-nitrone cycloadditions (SPANC) to yield N-alkylated isoxazolines.[34]
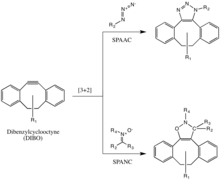
The SPAAC vs SpANC reaction
Because this reaction is metal-free and proceeds with fast kinetics (k2 equally fast as 60 one/Ms, faster than both the CuAAC or the SPAAC) SPANC can be used for live cell labeling. Moreover, substitution on both the carbon and nitrogen atoms of the nitrone dipole, and acyclic and endocyclic nitrones are all tolerated. This large allowance provides a lot of flexibility for nitrone handle or probe incorporation.[35]
However, the isoxazoline product is not equally stable as the triazole product of the CuAAC and the SpAAC, and tin can undergo rearrangements at biological conditions. Regardless, this reaction is still very useful as it has notably fast reaction kinetics.[34]
The applications of this reaction include labeling proteins containing serine as the first residue: the serine is oxidized to aldehyde with NaIO4 and so converted to nitrone with p-methoxybenzenethiol, N-methylhydroxylamine and p-ansidine, and finally incubated with cyclooctyne to give a click product. The SPANC too allows for multiplex labeling.[36] [37]
Reactions of strained alkenes [edit]
Strained alkenes besides employ strain-relief as a driving strength that allows for their participation in click reactions. Trans-cycloalkenes (usually cyclooctenes) and other strained alkenes such as oxanorbornadiene react in click reactions with a number of partners including azides, tetrazines and tetrazoles. These reaction partners can interact specifically with the strained alkene, staying bioorthogonal to endogenous alkenes found in lipids, fatty acids, cofactors and other natural products.[36]
Alkene and azide [3+2] cycloaddition [edit]
Oxanorbornadiene (or another activated alkene) reacts with azides, giving triazoles as a product. Still, these production triazoles are non aromatic as they are in the CuAAC or SPAAC reactions, and as a event are non equally stable. The activated double bond in oxanobornadiene makes a triazoline intermediate that subsequently spontaneously undergoes a retro Diels-alder reaction to release furan and give 1,two,3- or one,4,5-triazoles. Even though this reaction is slow, information technology is useful because oxabornodiene is relatively simple to synthesize. The reaction is not, withal, entirely chemoselective.[38]
Alkene and tetrazine inverse-demand Diels-Alder [edit]
![]()
A Tetrazine-Alkene reaction between a generalized tetrazine and a strained, trans-cyclooctene
Strained cyclooctenes and other activated alkenes react with tetrazines in an inverse electron-demand Diels-Alder followed by a retro [4+2] cycloaddition (see figure).[39] Like the other reactions of the trans-cyclooctene, ring strain release is a driving force for this reaction. Thus, iii-membered and 4-membered cycloalkenes, due to their high band strain, make ideal alkene substrates.[39]
Like to other [4+2] cycloadditions, electron-altruistic substituents on the dienophile and electron-withdrawing substituents on the diene advance the inverse-demand diels-alder. The diene, the tetrazine, past virtue of having the additional nitrogens, is a practiced diene for this reaction. The dienophile, the activated alkene, can often exist attached to electron-donating alkyl groups on target molecules, thus making the dienophile more suitable for the reaction.[40]
Alkene and tetrazole photoclick reaction [edit]
The tetrazole-alkene "photoclick" reaction is some other dipolar addition that Huisgen commencement introduced nigh 50 years ago (ChemBioChem 2007, eight, 1504. (68) Clovis, J. S.; Eckell, A.; Huisgen, R.; Sustmann, R. Chem. Ber. 1967, 100, threescore.) Tetrazoles with amino or styryl groups that can be activated past UV light at 365 nm (365 does not impairment cells) react quickly (then that the UV light does non have to be on for a long time, usually around 1–four minutes) to make fluorogenic pyrazoline products. This reaction scheme is well suited for the purpose of labeling in live cells, considering UV low-cal at 365 nm damages cells minimally. Moreover, the reaction proceeds quickly, and then that the UV light tin be administered for short durations. Breakthrough yields for short wavelength UV low-cal can exist college than 0.v. This allows tetrazoles to be used wavelength selectively in combination with some other photoligation reaction, where at the short wavelength the tetrazole ligation reaction proceeds virtually exclusively and at longer wavelength another reaction (ligation via o-quinodimethanes) proceeds exclusively.[41] Finally, the non-fluorogenic reactants requite rise to a fluorogenic product, equipping the reaction with a built-in spectrometry handle.
Both tetrazoles and the alkene groups have been incorporated as protein handles as unnatural amino acids, simply this benefit is not unique. Instead, the photoinducibility of the reaction makes it a prime candidate for spatiotemporal specificity in living systems. Challenges include the presence of endogenous alkenes, though normally cis (as in fatty acids) they can notwithstanding react with the activated tetrazole.[42]
Potential applications [edit]
The commercial potential of click chemistry is groovy. The fluorophore rhodamine has been coupled onto norbonene, and reacted with tetrazine in living systems.[43] In other cases, SPAAC betwixt a cyclooctyne-modified fluorophore and azide-tagged proteins allowed the selection of these proteins in prison cell lysates.[44]
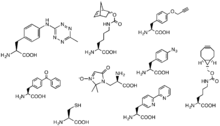
Methods for the incorporation of click reaction partners into systems in and ex vivo contribute to the scope of possible reactions. The development of unnatural amino acrid incorporation by ribosomes has immune for the incorporation of click reaction partners equally unnatural side groups on these unnatural amino acids. For example, an UAA with an azide side group provides convenient access for cycloalkynes to proteins tagged with this "AHA" unnatural amino acid.[45] In another instance, "CpK" has a side group including a cyclopropane alpha to an amide bond that serves as a reaction partner to tetrazine in an inverse diels-alder reaction.[46]
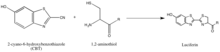
The synthesis of luciferin exemplifies another strategy of isolating reaction partners, which is to take advantage of rarely-occurring, natural groups such every bit the ane,2-aminothiol, which appears only when a cysteine is the final N' amino acid in a protein. Their natural selectivity and relative bioorthogonality is thus valuable in developing probes specific for these tags. The above reaction occurs between a ane,2-aminothiol and a 2-cyanobenzothiazole to brand luciferin, which is fluorescent. This luciferin fluorescence can exist and then quantified past spectrometry following a wash, and used to make up one's mind the relative presence of the molecule bearing the one,2-aminothiol. If the quantification of not-ane,2-aminothiol-bearing protein is desired, the protein of interest can be broken to yield a fragment with a N' Cys that is vulnerable to the ii-CBT.[47]
Boosted applications include:
- ii-dimensional gel electrophoresis separation[48]
- preparative organic synthesis of ane,4-substituted triazoles
- modification of peptide function with triazoles
- modification of natural products and pharmaceuticals
- natural product discovery [49]
- drug discovery
- macrocyclizations using Cu(I) catalyzed triazole couplings
- modification of DNA and nucleotides by triazole ligation
- supramolecular chemical science: calixarenes, rotaxanes, and catenanes
- dendrimer blueprint
- carbohydrate clusters and carbohydrate conjugation by Cu(1) catalyzed triazole ligation reactions
- polymers and biopolymers[fifty]
- surfaces[51]
- material science
- nanotechnology,[52]
- bioconjugation, for instance, azidocoumarin, and
- biomaterials[53]
In combination with combinatorial chemical science, high-throughput screening, and building chemic libraries, click chemistry has sped upwardly new drug discoveries past making each reaction in a multistep synthesis fast, efficient, and predictable.
Engineering license [edit]
The Scripps Inquiry Institute has a portfolio of click-chemical science patents.[54] Licensees include Invitrogen,[55] Allozyne,[56] Aileron,[57] Integrated Diagnostics,[58] and the biotech visitor baseclick,[59] a BASF spin-off created to sell products fabricated using click chemistry.[60] Moreover, baseclick holds a worldwide exclusive license for the research and diagnostic market for the nucleic acid field. Fluorescent azides and alkynes also produced past such companies equally Active Motif Chromeon[61] and Cyandye.[62]
See likewise [edit]
- Karl Barry Sharpless
- Rolf Huisgen
- Chemoproteomics
References [edit]
- ^ H. C. Kolb; M. G. Finn; K. B. Sharpless (2001). "Click Chemical science: Diverse Chemic Function from a Few Proficient Reactions". Angewandte Chemie International Edition. 40 (eleven): 2004–2021. doi:ten.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>three.0.CO;2-5. PMID 11433435.
- ^ R. A. Evans (2007). "The Ascent of Azide–Alkyne 1,iii-Dipolar 'Click' Cycloaddition and its Awarding to Polymer Science and Surface Modification". Australian Journal of Chemistry. 60 (half-dozen): 384–395. doi:10.1071/CH06457.
- ^ a b Stereolithography of polymer derived ceramics through Thiol-Ene Click Chemistry
- ^ Spiteri, Christian; Moses, John E. (2010). "Copper-Catalyzed Azide–Alkyne Cycloaddition: Regioselective Synthesis of 1,4,5-Trisubstituted 1,ii,3-Triazoles". Angewandte Chemie International Edition. 49 (one): 31–33. doi:10.1002/anie.200905322. PMID 19921729.
- ^ Hoyle, Charles Due east.; Bowman, Christopher Northward. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition. 49 (9): 1540–1573. doi:ten.1002/anie.200903924. PMID 20166107.
- ^ Lowe, A. B. Polymer Chemistry 2010, ane (ane), 17–36. DOI: ten.1039/B9PY00216B
- ^ Blackman, Melissa L.; Royzen Maksim; Flim-flam, Joseph G. (2008). "Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity". Journal of the American Chemical Society. 130 (41): 13518–13519. doi:ten.1021/ja8053805. PMC2653060. PMID 18798613.
- ^ Devaraj, Neal Yard.; Weissleder Ralph & Hilderbrand, Scott A. (2008). "Tetrazine Based Cycloadditions: Awarding to Pretargeted Alive Prison cell Labeling". Bioconjugate Chemical science. nineteen (12): 2297–2299. doi:x.1021/bc8004446. PMC2677645. PMID 19053305.
- ^ Stöckmann, Henning; Neves, Andre; Stairs, Shaun; Brindle, Kevin; Leeper, Finian (2011). "Exploring isonitrile-based click chemistry for ligation with biomolecules". Organic & Biomolecular Chemical science. 9 (21): 7303–5. doi:10.1039/C1OB06424J. PMID 21915395.
- ^ Kashemirov, Boris A.; Bala, Joy L. F.; Chen, Xiaolan; Ebetino, F. H.; Xia, Zhidao; Russell, R. Graham One thousand.; Coxon, Fraser P.; Roelofs, Anke J.; Rogers Michael J.; McKenna, Charles E. (2008). "Fluorescently labeled risedronate and related analogues: "magic linker" synthesis". Bioconjugate Chemistry. nineteen (12): 2308–2310. doi:10.1021/bc800369c. PMID 19032080.
- ^ Development and Applications of Click Chemical science Gregory C. Patton November viii, 2004 http://www.scs.uiuc.edu Online [ permanent dead link ]
- ^ Kolb, H.C.; Sharpless, B.M. (2003). "The growing touch on of click chemistry on drug discovery". Drug Discov Today. 8 (24): 1128–1137. doi:10.1016/S1359-6446(03)02933-7. PMID 14678739.
- ^ a b c L. Liang and D. Astruc: "The copper(I)-catalysed alkyne-azide cycloaddition (CuAAC) "click" reaction and its applications. An overview", 2011; 255, 23-24, 2933-2045, p. 2934
- ^ Rostovtsev, Vsevolod 5.; Light-green, Luke Grand; Fokin, Valery V.; Sharpless, One thousand. Barry (2002). "A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective "Ligation" of Azides and Terminal Alkynes". Angewandte Chemie International Edition. 41 (14): 2596–2599. doi:x.1002/1521-3773(20020715)41:14<2596::aid-anie2596>3.0.co;two-iv. PMID 12203546.
- ^ Tornoe, C. W.; Christensen, C.; Meldal, Yard. (2002). "Peptidotriazoles on Solid Stage: [i,2,iii]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides". Journal of Organic Chemistry. 67 (9): 3057–3064. doi:10.1021/jo011148j. PMID 11975567.
- ^ K. Wang, Ten. Bi, S. Xing, P. Liao, Z. Fang, X. Meng, Q. Zhang, Q. Liu, Y. Ji Light-green Chem., 13 (2011), p. 562
- ^ B. T. Worrell, J. A. Malik, V. V. Fokin 2013, 340, 457-459 ; J.E. Hein, V.Five. Fokin, Chem. Soc. Rev. 39 (2010) 1302.
- ^ Rodionov, Valentin O.; Fokin, Valery V.; Finn, M. Thou. (2005-04-08). "Mechanism of the Ligand-Free CuI-Catalyzed Azide–Alkyne Cycloaddition Reaction". Angewandte Chemie International Edition. 44 (15): 2210–2215. doi:10.1002/anie.200461496. ISSN 1521-3773. PMID 15693051.
- ^ Iacobucci, Claudio; Reale, Samantha; Gal, Jean-François; De Angelis, Francesco (2015-03-02). "Dinuclear Copper Intermediates in Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Directly Observed by Electrospray Ionization Mass Spectrometry". Angewandte Chemie International Edition. 54 (10): 3065–3068. doi:10.1002/anie.201410301. ISSN 1521-3773. PMID 25614295.
- ^ Özkılıç, Yılmaz; Tüzün, Nurcan Ş. (2016-08-22). "A DFT Study on the Binuclear CuAAC Reaction: Mechanism in Light of New Experiments". Organometallics. 35 (16): 2589–2599. doi:ten.1021/acs.organomet.6b00279. ISSN 0276-7333.
- ^ Ziegler, Micah Southward.; Lakshmi, K. V.; Tilley, T. Don (2017-04-nineteen). "Dicopper Cu(I)Cu(I) and Cu(I)Cu(II) Complexes in Copper-Catalyzed Azide–Alkyne Cycloaddition" (PDF). Journal of the American Chemic Club. 139 (xv): 5378–5386. doi:10.1021/jacs.6b13261. ISSN 0002-7863. PMID 28394586.
- ^ Brotherton, W. South.; Michaels, H. A.; Simmons, J. T.; Clark, R.J.; Dalal, N. South.; Zhu, Fifty. Org. Lett. 2009, 11, 4954.
- ^ Kuang, M.-C.; Michaels, H. A.; Simmons, J. T.; Clark, R. J.; Zhu, L" J. Org. Chem. 2010; 75, 6540.
- ^ Uttamapinant, C.; Tangpeerachaikul, A.; Grecian, S.; Clarke, S.; Singh, U.; Slade, P.; Gee, Yard. R.; Ting, A. Y" Angew. Chem. Int. Ed. 2012; 51, 5852
- ^ Alder, M.; Stein, G.; Finzenhagen, H. Justus Liebigs Ann.Chem 1931, 485, 211.
- ^ Alder, K.; Stein, G. Justus Liebigs Ann. Chem. 1933, 501, 1.
- ^ Wittig, G.; Krebs, A. Chem. Ber. 1961, 94, 3260.
- ^ Zhang, Li; Chen, Xinguo; Xue, Peng; Sun, Herman H. Y.; Williams, Ian D.; Sharpless, K. Barry; Fokin, Valery V.; Jia, Guochen (Nov 2005). "Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides". Periodical of the American Chemical Society. 127 (46): 15998–15999. doi:10.1021/ja054114s. PMID 16287266.
- ^ Huisgen, R. Angew. Chem. Int. Ed. Engl. 1963, two, 565
Agard, Northward. J.; Baskin, J. Chiliad.; Prescher, J. A.; Lo, A.; Bertozzi, C. R. (2006). "A Comparative Study of Bioorthogonal Reactions with Azides". ACS Chem. Biol. 1 (10): 644–648. doi:10.1021/cb6003228. PMID 17175580.
- ^ Agard, N. J.; Baskin, J. K.; Prescher, J. A.; Lo, A.; Bertozzi, C. R. (2006). "A Comparative Study of Bioorthogonal Reactions with Azides". ACS Chem. Biol. one (10): 644–648. doi:ten.1021/cb6003228. PMID 17175580.
- ^ Codelli, J. A.; Baskin, J. M.; Agard, N. J.; Bertozzi, C. R. (2008). "2d-Generation Difluorinated Cyclooctynes for Copper-Free Click Chemistry". J. Am. Chem. Soc. 130 (34): 11486–11493. doi:10.1021/ja803086r. PMC2646667. PMID 18680289.
- ^ Ning, X.; Guo, J.; Wolfert, M. A.; Boons, G.-J. (2008). "Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Gratis and Fast Huisgen Cycloadditions". Angew. Chem. Int. Ed. 47 (12): 2253–2255. doi:10.1002/anie.200705456. PMC2835304. PMID 18275058.
- ^ Gordon, C. G.; Mackey, J. 50.; Jewett, J. C.; Sletten, East. K.; Houk, K. N.; Bertozzi, C. R. (2012). "Reactivity of Biarylazacyclooctynones in Copper-Free Click Chemistry". J. Am. Chem. Soc. 134 (22): 9199–9208. doi:x.1021/ja3000936. PMC3368396. PMID 22553995.
- ^ a b MacKenzie, DA; Sherratt, AR; Chigrinova, Thou; Cheung, LL; Pezacki, JP (Aug 2014). "Strain-promoted cycloadditions involving nitrones and alkynes—rapid tunable reactions for bioorthogonal labeling". Curr Opin Chem Biol. 21: 81–8. doi:10.1016/j.cbpa.2014.05.023. PMID 25022431.
- ^ (64) (a) Ning, X.; Temming, R. P.; Dommerholt, J.; Guo, J.; Ania, D.B.; Debets, M. F.; Wolfert, Thou. A.; Boons, Grand.-J.; van Delft, F. L" Angew. Chem. Int. Ed. 2010; 49, 3065. (b) McKay, C. S.; Moran, J.; Pezacki, J. P. Chem. Commun. (Cambridge, U. Chiliad.) 2010, 46, 931. (c) Debets, Yard. F.; van Berkel, Due south. S.; Dommerholt, J.; Dirks, A. T. J.; Rutjes, F. P. J. T.; van Delft, F. L. Acc. Chem. Res. 2011, 44, 805. (d) McKay, C. S.; Chigrinova, M.; Blake, J. A.; Pezacki, J. P. Org. Biomol. Chem. 2012, 10, 3066.
- ^ a b Lang, K.; Mentum, J. (2014). "Bioorthogonal Reactions for Labeling Proteins". ACS Chem. Biol. 9 (ane): sixteen–twenty. doi:10.1021/cb4009292. PMID 24432752.
- ^ MacKenzie, DA; Pezacki, JP (2014). "Kinetics studies of rapid strain- promoted [iii+2] cycloadditions of nitrones with bicyclo[half-dozen.1.0]nonyne". Can J Chem. 92 (4): 337–340. doi:10.1139/cjc-2013-0577.
- ^ (67) (a) van Berkel, Southward. Due south.; Dirks, A. T. J.; Meeuwissen, S. A.; Pingen, D. L. L.; Boerman, O. C.; Laverman, P.; van Delft, F. Fifty.; Cornelissen, J. J. L. M.; Rutjes, F. P. J. T. ChemBioChem 2008, 9, 1805. (b) van Berkel, Due south. Southward.; Dirks, A. T. J.; Debets, Grand. F.; van Delft, F. Fifty.; Cornelissen, J. J. Fifty. M.; Nolte, R. J. M.; Rutjes, F. P. J. T. ChemBioChem 2007, 8, 150
- ^ a b Liu, Fang; Paton, Robert S.; Kim, Seonah; Liang, Yong; Houk, K. N. (2013). "Diels–Alder Reactivities of Strained and Unstrained Cycloalkenes with Normal and Changed-Electron-Demand Dienes: Activation Barriers and Distortion/Interaction Analysis". J. Am. Chem. Soc. 135 (41): 15642–15649. doi:10.1021/ja408437u. PMID 24044412.
- ^ Rieder, Ulrike; Luedtke, Nathan Due west. (25 August 2014). "Alkene-tetrazine ligation for imaging cellular Deoxyribonucleic acid". Angew Chem Int Ed Engl. 53 (35): 9168–9172. doi:10.1002/anie.201403580. PMID 24981416.
- ^ Menzel, January P.; Feist, Florian; Tuten, Bryan; Weil, Tanja; Blinco, James P.; Barner‐Kowollik, Christopher (2019). "Calorie-free-Controlled Orthogonal Covalent Bond Formation at Ii Different Wavelengths". Angewandte Chemie International Edition. 58 (22): 7470–7474. doi:10.1002/anie.201901275. PMID 30916368.
- ^ Ramil, Carlo P; Lin, Qing (August 2014). "Photoclick chemistry: a fluorogenic low-cal-triggered in vivo ligation reaction". Current Opinion in Chemical Biological science. 21: 89–95. doi:10.1016/j.cbpa.2014.05.024. PMC4149939. PMID 25022432.
- ^ Devaraj, Neal K.; Weissleder, Ralph; Hilderbrand, Scott A. (December 2008). "Tetrazine-based cycloadditions: application to pretargeted live cell imaging". Bioconjugate Chem. nineteen (12): 2297–2299. doi:10.1021/bc8004446. PMC2677645. PMID 19053305.
- ^ Ding, H.; Demple, B (2000). "Direct nitric oxide betoken transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator". Proc. Natl. Acad. Sci. U.S.A. 97 (10): 5146–5150. Bibcode:2000PNAS...97.5146D. doi:10.1073/pnas.97.10.5146. PMC25796. PMID 10805777.
- ^ Dieterich; et al. (2007). "Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging". Nature Protocols. 2 (3): 532–540. doi:10.1038/nprot.2007.52. PMID 17406607. S2CID 2833184.
- ^ Yu; et al. (2012). "Genetically Encoded Cyclopropene Directs Rapid, Photoclick-Chemistry-Mediated Poly peptide Labeling in Mammalian Cells". Angew Chem Int Ed Engl. 51 (42): 10600–10604. doi:10.1002/anie.201205352. PMC3517012. PMID 22997015.
- ^ (a) Liang, G.; Ren, H.; Rao, J. Nat. Chem. 2010, 2, 54. (b) Ren, H.; Xiao, F.; Zhan, Yard.; Kim, Y.-P.; Xie, H.; Xia, Z.; Rao, J. Angew.Chem., Int. Ed. 2009, 48, 9658.
- ^ Ilya A. Osterman; Alexey V. Ustinov; Denis V. Evdokimov; Vladimir A. Korshun; Petr Five. Sergiev; Marina V. Serebryakova; Irina A. Demina; Maria A. Galyamina; Vadim Chiliad. Govorun; Olga A. Dontsova (January 2013). "A nascent proteome report combining click chemistry with 2DE" (PDF). Proteomics. 13 (one): 17–21. doi:10.1002/pmic.201200393. PMID 23161590. S2CID 9002232. Archived from the original (PDF) on 2015-06-30. Retrieved 2015-02-11 .
- ^ Cox, Courtney L.; Tietz, Jonathan I.; Sokolowski, Karol; Melby, Joel O.; Doroghazi, James R.; Mitchell, Douglas A. (17 June 2014). "Nucleophilic 1,iv-Additions for Natural Product Discovery". ACS Chemical Biology. ix (nine): 2014–2022. doi:10.1021/cb500324n. PMC4168802. PMID 24937678.
- ^ Michael Floros; Alcides Leão; Suresh Narine (2014). "Vegetable Oil Derived Solvent, and Catalyst Gratuitous "Click Chemistry" Thermoplastic Polytriazoles". BioMed Research International. 2014: 1–xiv. doi:10.1155/2014/792901. PMC4085725. PMID 25032224.
- ^ London, Gábor; Chen, Kuang-Yen; Carroll, Gregory T.; Feringa, Ben L. (2013). "Towards Dynamic Command of Wettability by Using Functionalized Altitudinal Molecular Motors on Solid Surfaces". Chemistry: A European Periodical. xix (32): 10690–10697. doi:10.1002/chem.201300500. PMID 23784916.
- ^ John East. Moses; Adam D. Moorhouse (2007). "The growing applications of click chemistry". Chem. Soc. Rev. 36 (8): 1249–1262. doi:10.1039/b613014n. PMID 17619685.
- ^ Jean-François Lutz; Zoya Zarafshani (2008). "Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide–alkyne "click" chemistry". Advanced Drug Delivery Reviews. sixty (9): 958–970. doi:10.1016/j.addr.2008.02.004. PMID 18406491.
- ^ "Archived copy". Archived from the original on 2012-05-15. Retrieved 2012-06-05 .
{{cite web}}: CS1 maint: archived re-create equally title (link) - ^ "Archived re-create". Archived from the original on 2012-12-17. Retrieved 2012-06-05 .
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "Xconomy: Allozyne Licenses Scripps Chemistry". 2010-07-15.
- ^ "Xconomy: Aileron and Scripps Ink Bargain". 2010-11-30.
- ^ "Archived re-create". Archived from the original on 2012-04-30. Retrieved 2012-06-05 .
{{cite web}}: CS1 maint: archived copy equally title (link) - ^ "baseclick GmbH :: Nosotros enable nucleic acid labeling bioconjugation". baseclick GmbH . Retrieved 2022-03-21 .
- ^ http://world wide web.basf.com/group/pressrelease/P-ten-427 [ permanent dead link ]
- ^ http://world wide web.chromeon.com/
- ^ "CYANDYE". web.archive.org. 2018-10-03. Retrieved 2022-03-21 .
External links [edit]
- Click Chemistry: Short Review and Contempo Literature
- National Science Foundation: Feature "Going Alive with Click Chemistry."
- Chemical and Engineering News: Feature "In-Situ Click Chemical science."
- Chemical and Engineering News: Feature "Copper-free Click Chemistry"
- Metal-free click chemical science review
- Click Chemistry - a Chem Soc Rev themed issue highlighting the latest applications of click chemistry, guest edited past Thousand G Finn and Valery Fokin. Published by the Purple Order of Chemistry
Source: https://en.wikipedia.org/wiki/Click_chemistry
Enviar um comentário for "Review Paper on Azide-alkyne Click Chemistry of Polymers Chemical Reviews"